what color changes did you observe when you added sudan solution to corn oil?
1.nine: Biomolecule Detection
- Folio ID
- 36751
Learning Objectives
Goals:
- Employ indicators to detect characteristics of a solution.
- Apply indicators to make up one's mind contents of an unknown solution.
- Employing positive and negative controls to validate a test.
Student Learning Outcomes:
Upon completion of this lab, students volition be able to:
- Describe the backdrop of some important biomolecules.
- Explain important characteristics of proteins and carbohydrates.
- Perform tests to notice the presence of carbohydrates and proteins.
- Explain the importance of a control in biochemical tests.
- Use a biochemical test to place the presence of a molecule in an unknown solution.
INTRODUCTION
The Macromolecules of Life: Proteins, Carbohydrates, and Lipids
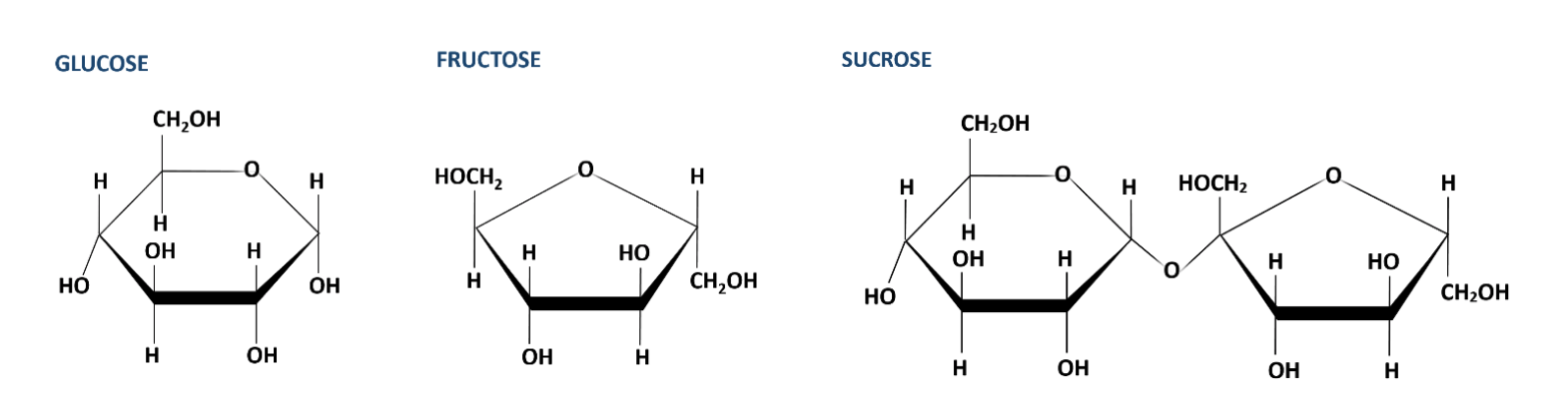
The cells of living organisms are composed of large molecules (macromolecules) sometimes as well referred to as organic molecules because of the presence of the chemical element carbon. Very many of the organic molecules found in living organisms are carbohydrates, proteins, lipids, and nucleic acids. Each of these macromolecules is made of smaller subunits. The different molecules take dissimilar chemical properties. For case, monosaccharides such equally glucose will react with a chemical amanuensis chosen Benedict'due south solution but disaccharides, like sucrose, and polysaccharides, similar starch will not. Similarly, proteins will react with a mix of potassium hydroxide and copper sulfate but gratis amino acids, carbohydrates, and lipids will not.
Today, we volition focus on iii of these molecular types: lipids, proteins and carbohydrates. You will work with nucleic acids in some other lab. Y'all may want to review the properties of the biomolecules of life.
| Molecular Blazon | Molecular Structure | Macro Construction |
|---|---|---|
| Sucrose | | Common source: Table carbohydrate |
| Starch | | Common source: Rice |
| Lipids | | Common source: Cooking oils |
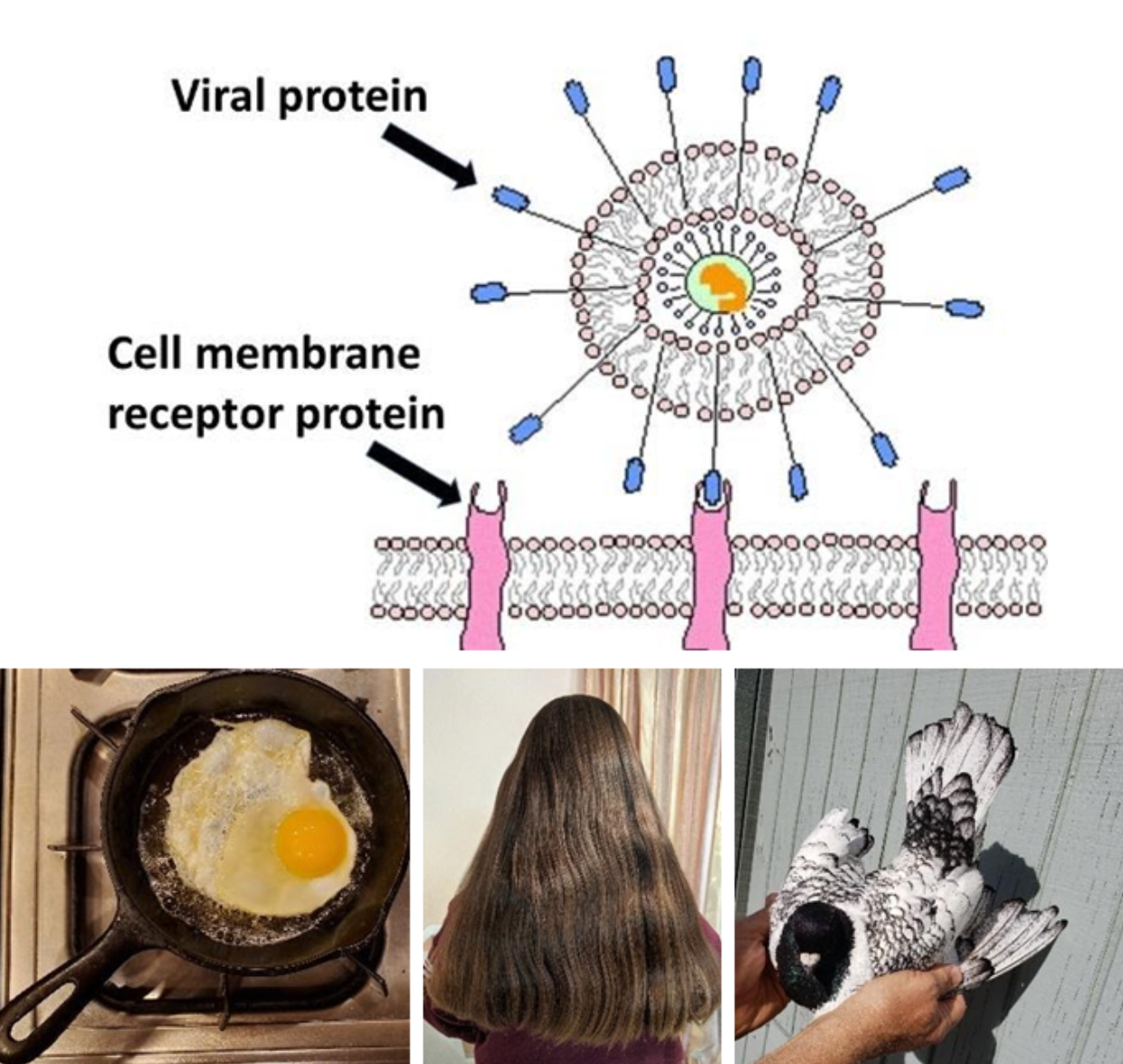
| Proteins | | Common sources: cell receptors, egg, hair, feathers |
Part I: Controlled Experiments to Identify Organic Compounds
Indicators are chemicals that change color when chemical conditions alter, such every bit pH, or when a chemical reaction takes place producing a colored molecule. There are many biochemical procedures that tin can exist used to notice the presence of important molecules. In this exercise, y'all will test diverse solutions in order to discover the presence of these molecules. Nosotros will employ controls every bit we test the solutions. Controls provide results to compare to the solution existence tested. Controls should give predictable results. By comparing the test solution consequence with the controls, y'all can determine the effect of the test solution.
A positive control contains the variable for which you are testing. When the positive control is tested, it reacts in an expected manner. If, for example, you are testing for a type of sugar in unknown solutions, then an advisable positive control is a solution known to contain that type of carbohydrate. The resulting reaction, when properly performed, volition demonstrate that the reagents work as expected and shows what the upshot should await similar if the exam solution is positive. If the positive control does not react every bit expected, your test is not valid. Perhaps your test reagents are not working properly.
A negative command does non comprise the variable for which you are testing. Often a negative control contains only water. It will non react with the indicator reagents. Similar the positive control, the negative control solution shows you lot what a negative event looks similar and verifies that the detecting reagent is working properly. If the negative control does react, your exam result is not valid. Perhaps the command solution or reaction tube was contaminated with the test variable.
I. Carbohydrates
Benedict's Examination for Monosaccharides
Molecules made of the atoms carbon (C), hydrogen (H), and oxygen (O), in a ratio of 1:ii:one are carbohydrates. For example, glucose, one of the most of import carbohydrates for living cells, has the chemic formula C6H12O6. Elementary sugars as well known as monosaccharides are carbohydrates. Paired monosaccharides class disaccharides. A common example of a disaccharide is the tabular array sugar, sucrose. It is composed of the monosaccharides glucose and fructose linked to fructose. Similarly, linking three or more monosaccharides forms a polysaccharide. Starch, glycogen, or cellulose are polysaccharides of import to cells and have many monomers of glucose linked together in different means.
Benedict'southward reagent is the indicator nosotros use to detect monosaccharides. When monosaccharides are mixed with Benedict'southward and heated, a colour change occurs. If there is a small amount of monosaccharide in the solutions, a greenish solution is produced. If the solution contains a big amount of monosaccharide, an orange precipitate results. A precipitating solution ways minor particles settle out of the solution.
Monosaccharides + Bridegroom'southward reagent + Heat ⇒ Green to Orange
II. Proteins
The cell relies on proteins for very many functional reasons. Proteins may exist enzyme catalysts, grade channels for molecules to pass across membranes, form structures and more. The subunit of protein molecules are monomers of amino acids. The bond that forms between amino acids to form poly peptide is called a peptide bail.
Peptide bonds tin exist detected by using two chemic reagents, potassium hydroxide (KOH) and copper sulfate (CuSO4). Potassium hydroxide causes a protein to break apart and then that copper sulfate tin react with the peptide bonds. The resulting color is imperial. The more protein, and hence more peptide bonds, in the solution, the darker the resulting majestic will become.
Testing for Monosaccharides with Bridegroom's Reagent
Proteins + KOH + CuSO4 ⇒ Purple
Materials
- Test tubes labeled with the contents you will add to each tube
- Beaker with water and hot plate (water heated to most boiling)
- Metric ruler
- Marker
- Deionized h2o and carbohydrate solutions
- Appropriate tool to remove hot tubes from water
Process
- Obtain 5 test tubes and number them one – five.
- Employ a mark to bespeak 2.5 cm from the bottom and another mark at 5cm from the bottom.
- Fill each test tube to your 2.5 cm mark with the advisable solution:
1. Distilled water 2. Concentrated glucose solution 3. Diluted glucose solution 4. Sucrose solution 5. Starch solution - Add Bridegroom's solution to each tube to the v cm mark.
- Place all of the tubes in a hot (xc°C) water-bath for 2 min, and discover color-changes during this time.
- Afterward 2 min, remove the tubes from the water-bath and tape the color of their contents in the table below. Also observe your classmate'due south reactions.
Observations
Perform the Benedict's examination for monosaccharides. Reproduce this tabular array in your lab book and complete it with your observations.
| Tube Contents | Colour after reaction | Presence of monosaccharide? |
|---|---|---|
| 1. Water | ||
| 2. Concentrated glucose | ||
| 3. Diluted glucose | ||
| iv. Sucrose solution | ||
| 5. Starch solution |
Instructions to clean upward
* Clean tubes are very important. Contaminated tubes may influence results of hereafter tests.
1. When your observations are complete, carefully wash and rinse the tubes post-obit the instructions in part 2. Y'all may leave the markings on them until the terminal make clean up process of the day.
Information Analysis
- Which of the above solutions serve as your positive command? Negative control?
- Examine your test and your classmates test solutions. Which solutions were positive for monosaccharides?
- Which contains a college concentration of monosaccharides, spud juice or onion juice? How practise you know?
- Which solutions did not react with the Benedict's solution?
Testing for Peptide Bonds (Protein)
Materials
- Four clean test tubes labeled with the contents yous will add to each tube
- deionized water, and exam solutions
- Indicator reagents potassium hydroxide (KOH) and copper sulfate (CuSO 4 )
Procedure
Perform the Peptide Bail test for Poly peptide
 Exercise not spill the KOH – it is extremely caustic. Rinse your skin if it comes in contact with KOH.
Exercise not spill the KOH – it is extremely caustic. Rinse your skin if it comes in contact with KOH.
- Use your four clean test tubes from the previous procedure. They still demand to be numbered and marked at 2.5 and 5 cm from the bottom.
- Fill each test tube to the 2.five cm mark with the appropriate solutions indicated below
- Water
- Protein Solution
- Amino Acid Solution
- Examination Solution
- Add potassium hydroxide (KOH) to the 5cm mark on each test tube.
- Add five drops of copper sulfate (CuSOiv) to tube and mix well.
- Record the color of the tubes' contents in the tabular array below. Too observe your classmate's reactions.
- When finished dump the contents of the tubes and wash them. Rinse with distilled water.
Observations
Perform the Protein Exam: Reproduce this table in your lab book and complete it with your observations.
| Tube Contents | Color after reaction | Presence of protein? |
|---|---|---|
| Water | ||
| Protein solution | ||
| Amino acid solution | ||
| Unknown solution |
Instructions to clean up
* Clean tubes are very of import. Contaminated tubes may influence results of future tests.
When your observations are complete, carefully wash and rinse the tubes following the instructions in Part I.
Data Analysis
- Which of the solutions is a positive control? Which is a negative control?
- Exercise individual amino acids have peptide bonds? How do you lot know this to be true?
- What type of solution did you test equally your unknown? Did information technology contain protein?
- Find your classmates reactions and describe which unknown solutions contain the near and the to the lowest degree poly peptide. How can you tell?
Three. Lipids
Lipids are a grade of molecules that are not soluble (do not dissolve) in h2o. They are composed of the molecular building blocks of glycerol and three fatty acids. Fatty acids come up in two major types, saturated and unsaturated. This difference is due to the presence of item types of bonds within the fatty acid molecule (see figure) and affect the shape and characteristics of the overall lipid containing these fatty acids. You may want a review of lipids.
Testing for Lipid with Sudan IV
 Apply gloves and avert contact with Sudan Four equally it is considered a possible carcinogen. Immediately wash your pare with soap and enough of water if yous come in contact with the solution.
Apply gloves and avert contact with Sudan Four equally it is considered a possible carcinogen. Immediately wash your pare with soap and enough of water if yous come in contact with the solution.
Materials
- Filter paper (small enough to fit in the petri dish) and pencil with areas labeled for test substances
- clean empty petri dish
- solution of 0.2% Sudan IV
- Gloves (see safe warning)
- Dedicated transfer pipettes or micropipettes with tips.
- Solutions of deionized h2o, vegetable oil, and exam solutions (foam, dairy milks, coconut milk, soy milk etc.)
- optional- hairdryer
Procedure
- Obtain filter paper and on the far border marker with pencil which solutions will be placed toward the interior of the mark.
- Drop a small amount of solution most the appropriate mark. 1. Distilled water ii. Vegetable oil 3-6. Examination solutions
- Allow to dry. Use a hairdryer to speed up this process.
- While the paper is drying, answer the Data Analysis questions below.
- Soak the paper in the petri dish containing 0.two% Sudan 4. (handle with gloved hands)
- Rinse the newspaper in distilled h2o and allow to dry out.
- Record the color of the spots in the table beneath. Also notice your classmate's reactions.
Observations
Sudan Four test for lipid: Reproduce this table in your lab book and consummate it with your observations. The darker the stain, the more lipid is present.
| Spot Contents | Color afterward reaction | Relative amount of lipid? |
|---|---|---|
| i. Water | ||
| two. Vegetable oil | ||
| 3. | ||
| 4. | ||
| five. | ||
| 6. |
Instructions to clean up:
When your observations are complete, carefully dispose of any remaining Sudan Four solution in the container provided by your instructor. Always use gloves and exercise not move the container if there is a danger of spilling.
Information Assay
- Which of the above solutions serve as your positive command? Your negative control?
- Hypothesize which solutions volition contain the greatest amount of lipid. Why do you believe this to be true?
- Which solutions contained the greatest amount of lipid?
- Did your observations support your hypothesis? Were you surprised by some of the results? Explain.
Part II. The Saga of the Soda Dispenser
Enrique was a new employee. This was his first job and he had only been on the job for a couple of weeks and was all the same on "hiring probation." He liked the crew he worked with and the paycheck that would come every few weeks. He wanted to stay. Today, in that location was a problem and he had to figure out something fast to solve it. He knew that if he did, the manager would be really pleased and his job was guaranteed.
Someone was lament that the soda dispenser was dispensing "regular" cola from the "diet cola" dispenser. The customer claimed to exist on a reduced-calorie diet and was not happy about the extra calories consumed. There was more at stake than one unhappy customer, though. The manager told Enrique that many of their customers were diabetic and consuming carbohydrate-laden soda could change their blood-sugar chemistry in a unsafe manner. They could not let those customers to be harmed.
Telescopic of the Trouble
If the diet soda dispenser did have regular soda, then did the regular soda dispenser have nutrition? What almost the Dr. Pepper dispenser? That, at least, tasted like Dr. Pepper, so information technology was OK- or was it? What a mess! Should they throw all the soda in the dispenser out and start again? Or was there some way of determining if the soda was being dispensed correctly? If they could make up one's mind what the trouble was, they could relieve the business money and not waste product the soda products.
Enrique'due south Attempt to Solve the Mystery
Enrique knew that most soda had loftier fructose corn syrup in information technology simply nutrition soda had sugar substitutes in it: Substitutes that were not sugar but fooled your taste buds into believing information technology was.
Questions for your lab volume:
- Does the regular soda have loftier fructose corn syrup in it? Look at the label decide if it does or doesn't. Write your observation in your lab book.
- Does the diet soda have high fructose corn syrup in it? Expect at the label determine if it does or doesn't. Write your ascertainment in your lab book.
- Determine whether fructose is a monosaccharide, disaccharide or polysaccharide.
- Tin we do a test?
Just the other day, in science lab, Enrique had run some tests on solutions in lodge to make up one's mind their compositions. 1 of the tests was for detecting monosaccharides in solution! He knew his science teacher would withal be in the classroom at this time and the schoolhouse was barely a 5 minute walk from the eatery. He could solve the mystery in under 30 minutes! Enrique quickly told his manager his plan and grabbed some cups of soda, which he labeled, and then he could tell which dispenser they came from, and then headed out. Enrique chop-chop ran to the school lab and got permission to run his experiment. Assist Enrique gear up an experiment to test the soda.
More questions for your lab volume:
- Would it exist a good idea to include controls? If then, which solutions?
- Which detector reagent(southward) will you use?
- What colors will you look for to bespeak the presence of the "regular" soda?
- How many test tubes do you demand? How will you lot characterization them?
Testing Unknown Soda Solutions
Materials
- Clean exam tubes labeled with the contents y'all volition add to each tube
- deionized water, and solutions to test
- Indicator
Procedure
Perform the test for monosaccharides:
- Obtain the needed number of make clean test tubes and marker them at two.5 and v cm as earlier. Code them as to the contents (numbers respective to your solutions- which you lot tape below)
- Obtain the unknown solutions from your instructor.
- Fill the tubes to the 2.five cm marker with the command and examination substances.
- Fill the tubes to the five cm marking with indicator and treat was needed.
- Reproduce this table in your lab book and complete information technology with your observations, then answer the questions regarding the soda saga.
Observations
Perform the Appropriate Test: Reproduce this table in your lab book and complete it with your observations.
| Tube Contents | Colour subsequently reaction | Presence of fructose? | Diet or regular? |
|---|---|---|---|
| i. | |||
| two. | |||
| three. | |||
| four. | |||
| 5. | |||
| 6. |
Instructions to clean upwards:
 Practice NOT allow ethanol to come up in contact with the hotplate. Ethanol is very flammable.
Practice NOT allow ethanol to come up in contact with the hotplate. Ethanol is very flammable.
*Clean tubes are very important. Contaminated tubes may influence results of future tests.
- When your observations are complete, advisedly wash and rinse the tubes following the instructions in part 1.
- At the end of the lab catamenia be sure all labels are removed from the tubes using a small piece of newspaper towel and ethanol.
Final Conclusion
- What does Enrique tell his manager? Is the soda dispenser messed upwards or not?
- What, if whatever, soda needs to be changed?
Study Questions
- Why should you lot always include controls in each process?
- What serves as a good negative command and why?
- Describe a positive control.
- If you run a test for monosaccharide on what you believe is "regular" lemon lime-flavored soda, but the solution is sky-bluish later on heating with Bridegroom's what does this tell you?
- What if just Later on running your exam, yous read the label of the lemon-lime soda and find that the ingredients do not contain fructose but does contain sucrose. Is your exam procedure faulty or is in that location another caption for your result?
Attributions
- Sucrose Molecular Construction from LibreTexts v.2 Carbohydrates.
- Poly peptide Structure diagram by Lady of Hats, Public Domain, via Wikimedia Commons.
- Amino Acids forming a peptide bond (bottom image) by OpenLab at CitiTech CC-BY-NC-SA
Source: https://bio.libretexts.org/Bookshelves/Biotechnology/Lab_Manual%3A_Introduction_to_Biotechnology/01%3A_Techniques/1.09%3A_Biomolecule_Detection
0 Response to "what color changes did you observe when you added sudan solution to corn oil?"
Post a Comment